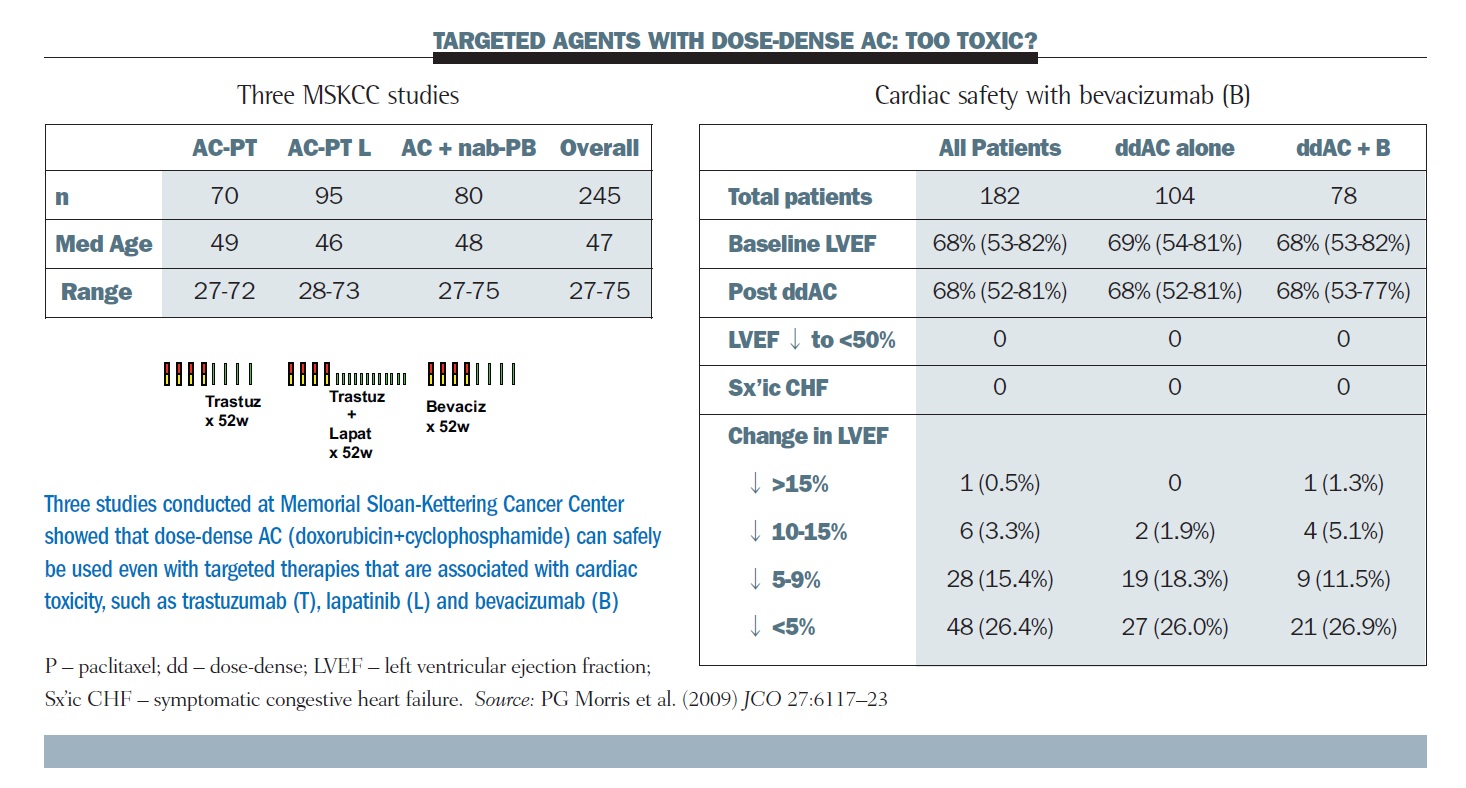
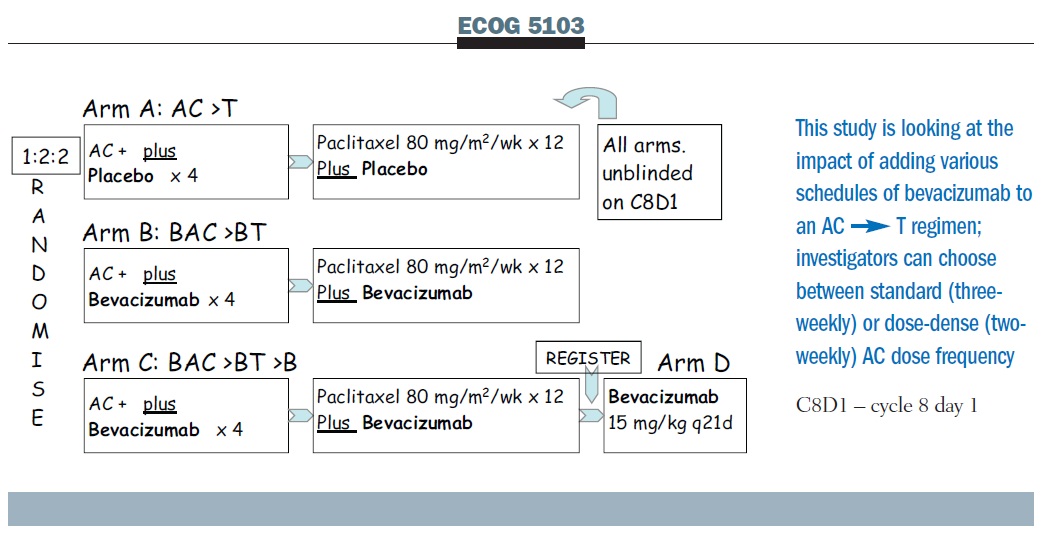
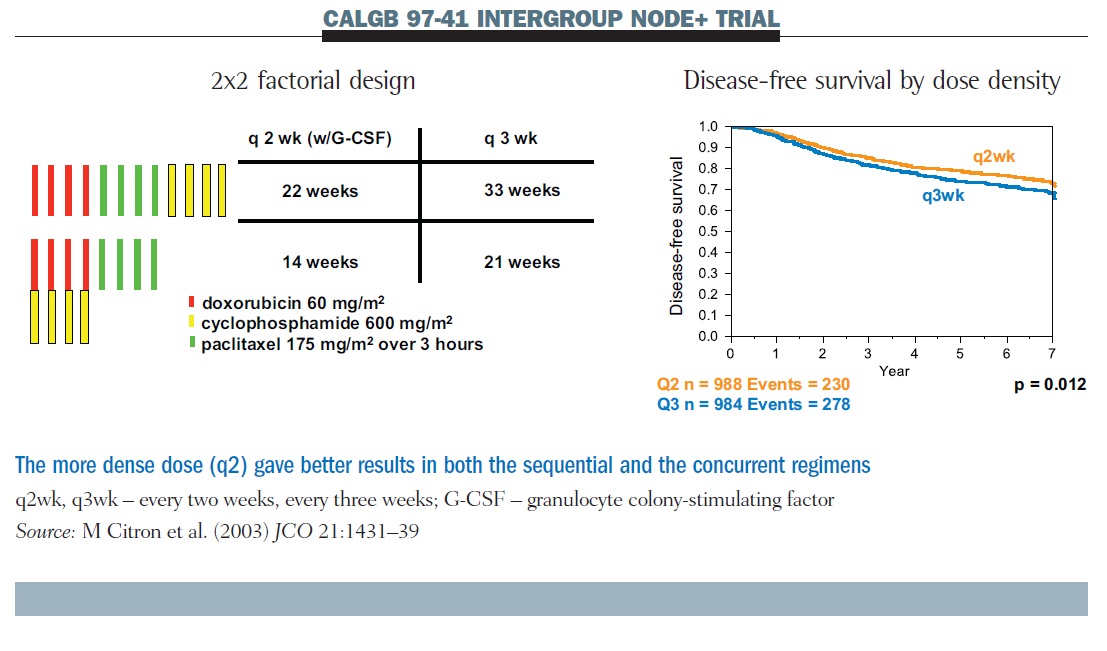
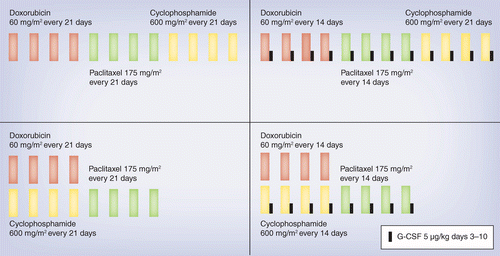
SWOG S0800 (NCI CDR0000636131): addition of bevacizumab to neoadjuvant nab-paclitaxel with dose-dense doxorubicin and cyclophosp

Four cycles of docetaxel and cyclophosphamide as adjuvant chemotherapy in node negative breast cancer: A real-world study - ScienceDirect

Anthracycline-free or short-term regimen as adjuvant chemotherapy for operable breast cancer: A phase III randomized non-inferiority trial - The Lancet Regional Health – Western Pacific
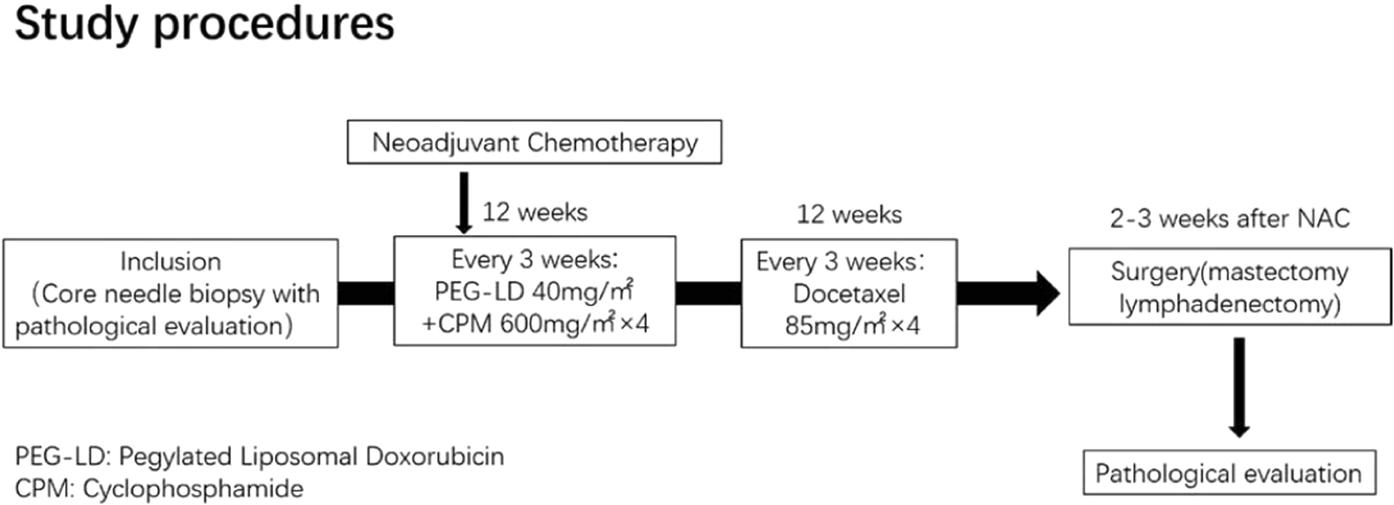
Pegylated liposomal doxorubicin plus cyclophosphamide followed by docetaxel as neoadjuvant chemotherapy in locally advanced breast cancer (registration number: ChiCTR1900023052) | Scientific Reports

Standardizing Chemotherapy Regimen Nomenclature: A Proposal and Evaluation of the HemOnc and National Cancer Institute Thesaurus Regimen Content | JCO Clinical Cancer Informatics

Assessment of CBC Changes in Breast Cancer Patients Following Treatment with 5-Flourouracil, Adriamycin and Cyclophosphamide (FAC-Protocol) and Adriamycin and Cyclophosphamide (AC-Protocol) | Semantic Scholar
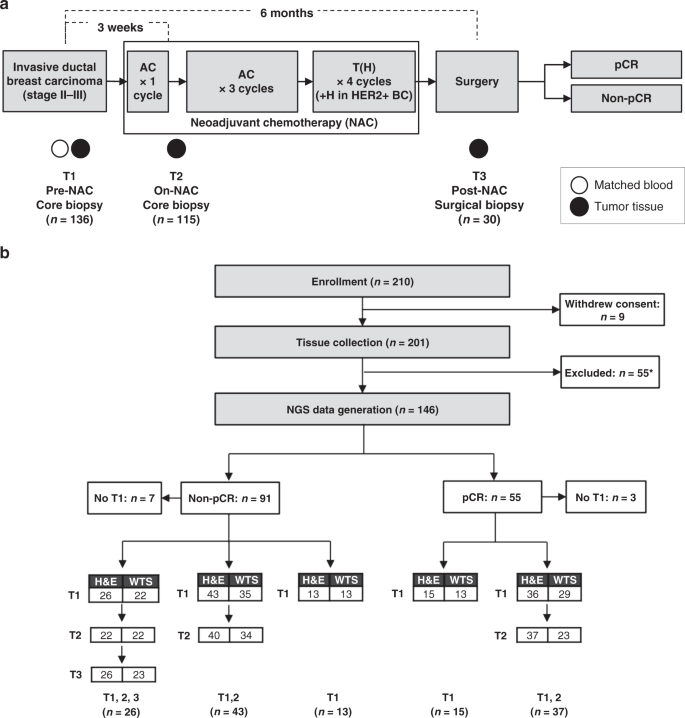
Chemotherapy induces dynamic immune responses in breast cancers that impact treatment outcome | Nature Communications

PDF) Dose Dense Doxorubicin and Cyclophosphamide Followed by Paclitaxel (Dose Dense AC Followed by T) Regimen for Breast Cancer

Standardizing Chemotherapy Regimen Nomenclature: A Proposal and Evaluation of the HemOnc and National Cancer Institute Thesaurus Regimen Content | JCO Clinical Cancer Informatics

CONSORT diagram for Alliance/Cancer and Leukemia Group B 49907 trial.... | Download Scientific Diagram

Standardizing Chemotherapy Regimen Nomenclature: A Proposal and Evaluation of the HemOnc and National Cancer Institute Thesaurus Regimen Content | JCO Clinical Cancer Informatics

Phase 2 Study of Dose-Dense Doxorubicin and Cyclophosphamide Followed by Eribulin Mesylate With or Without Prophylactic Growth Factor for Adjuvant Treatment of Early-Stage Human Epidermal Growth Factor Receptor 2–Negative Breast Cancer -